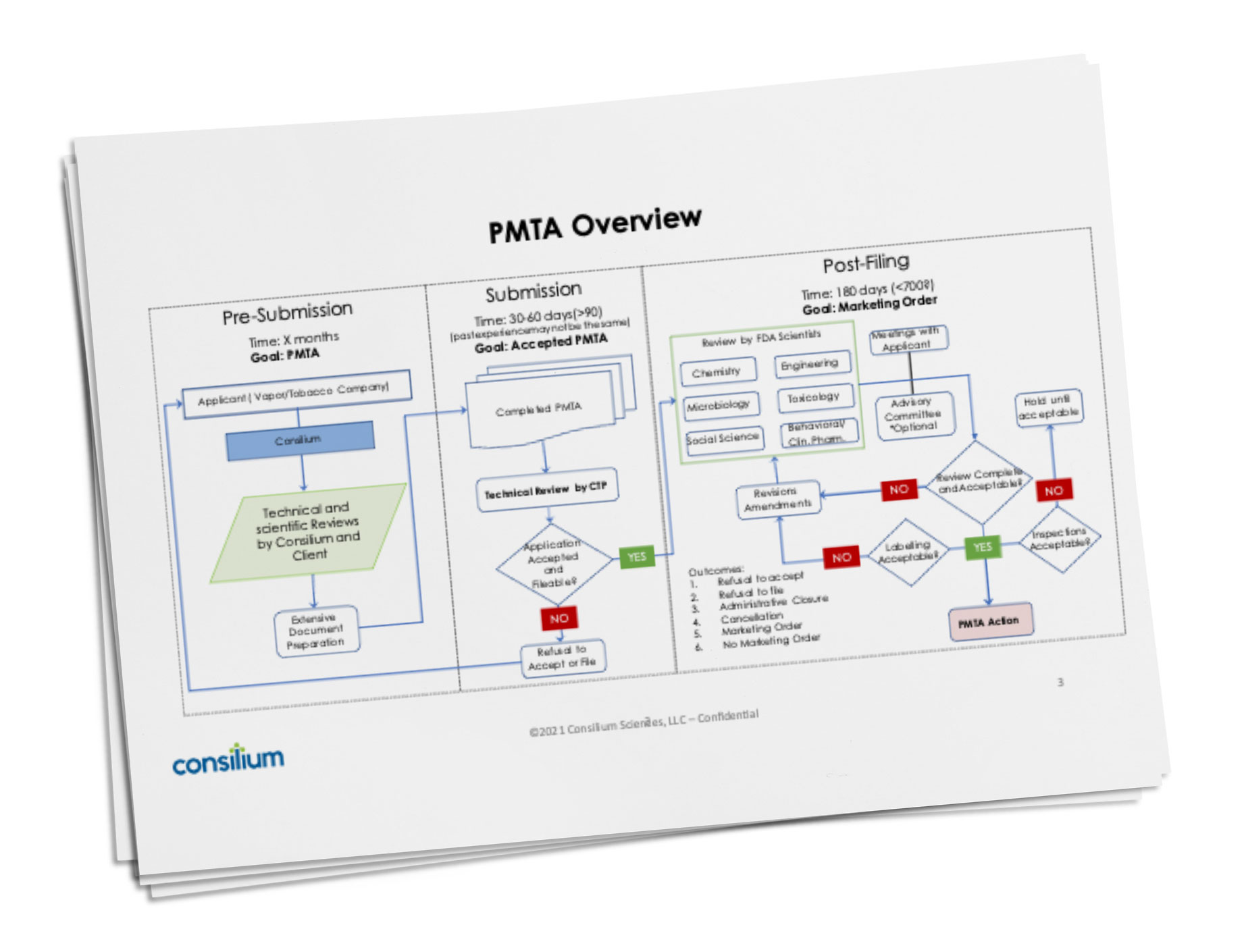
PMTAs and other regulatory applications can appear very complex. We have learned that experience is the best teacher when it comes to developing, preparing and submitting these documents, and Consilium has substantial PMTA, SE, MRTPA and TPMF experience. Shown below is a very condensed overview of the three steps to a PMTA – Pre-Submission, Submission, and Post-Filing. Consilium also has a detailed process template for each of these three steps which we use to develop a custom process-flow for each PMTA.
How we coordinate content creation and publication
Consilium has developed a robust system for developing client-specific regulatory strategy and generating submission-ready PMTAs, from information collection, scientific study design, and data analysis through copy-editing, quality control, and publishing.
Acceptance and Filing Reviews
What happens after submission of a PMTA? This flowchart walks through the Acceptance Review and Filing Review processes that occur prior to Substantive Review. Consilium is with you every step of the way
Scientific Review
After making it through Acceptance and Filing Review, a PMTA enters Substantive Review. At this step, FDA Subject Matter Experts review and evaluate the submitted information to determine whether the product under review is Appropriate for the Protection of Public Health (APPH). Consilium is prepared to quickly respond to FDA deficiency questions and design studies that may be required to sufficiently answer those questions.
Marketing Denial Order
FDA has outlined options to remedy deficiencies listed in a Marketing Denial Order. These pathways allow resubmission without completely starting over. A resubmission to address deficiencies may be eligible for an abbreviated PMTA review process.
Marketing Granted Order
“Congratulations, you received a Marketing Granted Order!” Are you prepared to meet the requirements to begin or continue selling your product? Consilium is with you every step of the way and can support your post market surveillance needs.
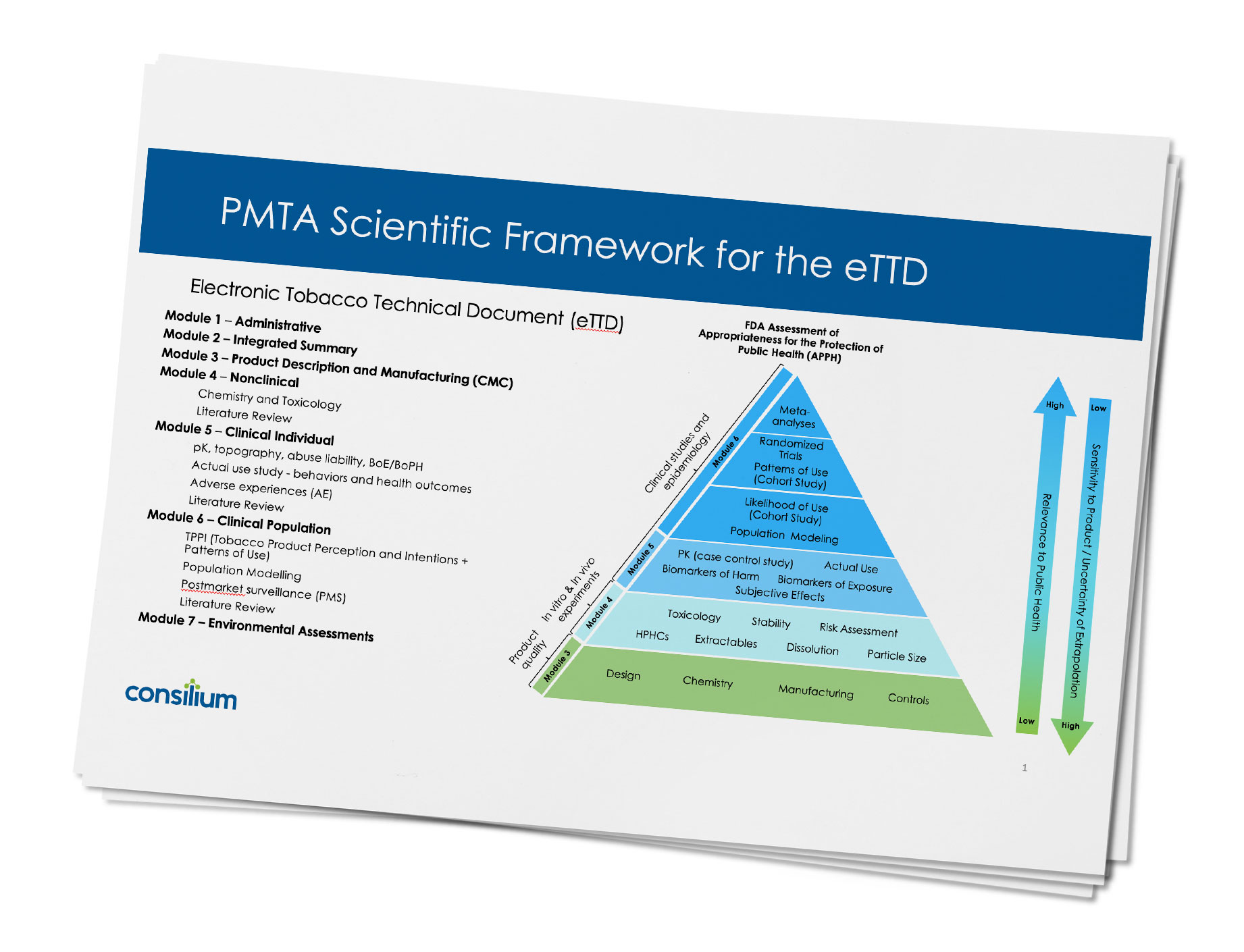
The FDA plans to require the standardized Electronic Tobacco Technical Document (eTTD) publishing format, which is briefly summarized below. Although there are only seven modules to a PMTA application, each of the modules can be lengthy and complex. Consilium has worked on numerous PMTAs utilizing eTTD publishing format and is quite familiar with different tobacco product categories.